Diamond and Graphite
Introduction
Diamond and graphite are both crystalline forms of the element carbon. You might expect them to share many traits given their common building block, but they're actually worlds apart. This difference sheds light on key aspects of crystals in general.
Both consist purely of carbon atoms, yet their behaviors couldn't be more contrasting. Let's dive into what sets them apart.
Comparing Their Properties
Diamond ranks as the hardest mineral known, scoring a perfect 10 on the Mohs scale, while graphite is among the softest, coming in below 1. This makes diamond ideal for cutting and grinding, and graphite perfect for lubrication.
Diamond acts as an electrical insulator, but graphite conducts electricity well. Typically, diamond is clear and transparent, whereas graphite appears dark and opaque.
And talk about value - diamond commands high prices in jewelry, but graphite is so affordable it's used in everyday pencil leads. Still, graphite holds its own in fascinating ways, as we'll see. If you're interested in learning more about this remarkable gemstone, explore our detailed diamond information guide.
Understanding Atomic Structures
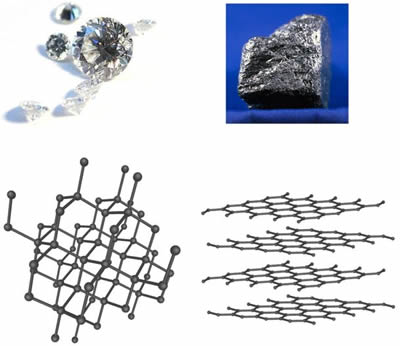
The key to their differences? It's all in how the atoms arrange themselves. Graphite forms in flat layers where carbon atoms bond strongly within each sheet but weakly between them. Diamond, however, features a three-dimensional network where each carbon atom links firmly to four others, creating a rigid, packed structure.
In graphite, those weak interlayer bonds explain its softness - layers slide easily. But roll those sheets into fibers and twist them into threads, and the inner strength shines through, especially when bound with resins like epoxy.
Real-World Applications
Diamond's toughness makes it invaluable for industrial tools, but graphite surprises with its high-tech roles. It's the strength booster in composites for cars, planes, premium golf clubs, and tennis racquets. These materials boast impressive strength-to-weight ratios, proving graphite's versatility beyond pencils.
Ever wonder how something so soft ends up in aircraft? It's because we harness its layered strength in engineered forms, turning potential weakness into an asset.
The Role of Crystal Structure
The contrast between diamond and graphite underscores how crystal structure dictates a gemstone's traits. For instance, gems with cubic crystals - like diamond, garnet, and spinel - share single refraction, bending light uniformly in all directions due to their symmetry.
Other crystal systems, such as orthorhombic, monoclinic, and triclinic, are doubly refractive because of their unique internal axes and symmetries that alter light paths differently. Beyond these scientific aspects, you can also discover the symbolic meaning and healing powers of diamond. Note: Claims about healing or spiritual properties are based on traditional beliefs and are not scientifically proven. They should not replace professional medical advice.
Frequently Asked Questions
What are diamond and graphite?
Both are forms of pure carbon, but they differ in crystal structure, leading to vastly different properties.
Why is diamond hard while graphite is soft?
Diamond has a three-dimensional network of strong bonds, whereas graphite's bonds are strong within layers but weak between them, allowing easy slippage.
How is graphite used in high-tech applications?
Its layers can be formed into strong fibers and composites, providing high strength-to-weight ratios for items like aircraft and sports equipment.
What does crystal structure mean for gem properties?
It influences hardness, refraction, conductivity, and more, as seen in how cubic crystals like diamond are singly refractive.

