Synthetic Ruby & Sapphire
 The ability to create gemstones in the laboratory began in 1902 when Auguste Verneuil, a French chemist, developed a method known as flame fusion to create synthetic ruby. This was no mere laboratory experiment. Verneuil soon expanded his lab into a full scale production facility and was turning out 1,000 kg of synthetic corundum annually by 1907. The ability to create gemstones in the laboratory began in 1902 when Auguste Verneuil, a French chemist, developed a method known as flame fusion to create synthetic ruby. This was no mere laboratory experiment. Verneuil soon expanded his lab into a full scale production facility and was turning out 1,000 kg of synthetic corundum annually by 1907.
Verneuil developed flame fusion primarily for the synthesis of ruby, but the same method can be used for the creation of other gem materials, including sapphire, star sapphire, spinel, rutile and strontium titanate.
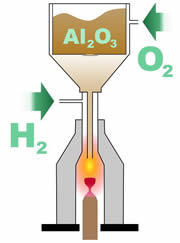
The basic concept of the flame fusion method is to take the raw gem material, melt it, and then allow it to recrystallize. The concept is simple, but this method depended on some key bits of technology. First, it was necessary to have extremely pure material to begin the process with. In the case of loose ruby or loose sapphire, this meant aluminum oxide that was free from impurities such as sodium. The earliest attempts to recrystallize ruby were actually accomplished by melting natural ruby crystals, due to the lack of fine, pure aluminum oxide.
 Next, it was necessary to have a way to heat the powdered aluminum oxide to a temperature of at least 2,000 degrees Celsius. The recently developed oxyhydrogen torch provided Verneuil with the technology for his furnace. Next, it was necessary to have a way to heat the powdered aluminum oxide to a temperature of at least 2,000 degrees Celsius. The recently developed oxyhydrogen torch provided Verneuil with the technology for his furnace.
During the Verneuil process, the powdered aluminum oxide is released down a tube where it passes through a flame that melts the material into small droplets. These droplets fall onto an earthen support rod placed at the bottom of the furnace.
The droplets form a single crystal called a boule. The boule has a characteristic cylindral shape with a tapered end. It is usually about 13 to 25 millimeters in diameter, 50 to 100 millimeters long, and weighs 75 to 250 carats.
Crystals produced by the Verneuil flame fusion process are chemically and physically equivalent to naturally occurring crystals, and strong magnification is usually required to distinguish between the two. One of the signature characteristics of a Verneuil crystal is curved growth lines, which form as the cylindrical boule grows upwards in an environment with a high thermal gradient. The equivalent growth lines in natural crystals are parallel.
 Another distinguishing feature of gems produced by flame fusion is the frequent presence of microscopic gas bubbles formed due to excess oxygen in the furnace. Imperfections in natural crystals are usually solid impurities. However, gas bubbles alone can no longer be taken as a reliable indication of a synthetic ruby; natural rubies that have been fracture-filled with lead-glass often exhibit gas bubbles in the glass-filled cavities. But please note that the fracture-filled rubies are typically heavily included while the flame fusion rubies have no natural inclusions. Another distinguishing feature of gems produced by flame fusion is the frequent presence of microscopic gas bubbles formed due to excess oxygen in the furnace. Imperfections in natural crystals are usually solid impurities. However, gas bubbles alone can no longer be taken as a reliable indication of a synthetic ruby; natural rubies that have been fracture-filled with lead-glass often exhibit gas bubbles in the glass-filled cavities. But please note that the fracture-filled rubies are typically heavily included while the flame fusion rubies have no natural inclusions.
|
